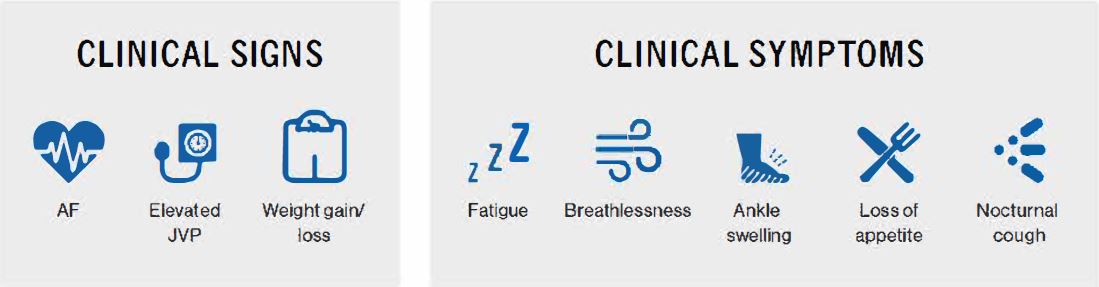
Heart Failure (HF) is defined by the European Society of Cardiology as a clinical syndrome characterised by symptoms such as breathlessness, ankle swelling and fatigue that may be accompanied by signs such as elevated jugular venous pressure, pulmonary crackles and peripheral oedema. HF is caused by a structural and/or functional cardiac abnormality, and results in a reduced cardiac output and/or elevated intracardiac pressures at rest or during stress.1
The main terminology used to describe HF is based on measurement of left ventricular ejection fraction (LVEF).1 Differentiating patients this way is important due to different underlying aetiologies, demographics, co-morbidities and response to therapies.1
HF with preserved ejection fraction (HFpEF)
Normal LVEF, typically considered as ≥50%1
HF with mildly reduced ejection fraction (HFmrEF)
Moderately reduced LVEF, 41–49%1
HF with reduced ejection fraction (HFrEF)
Reduced LVEF, typically considered as ≤40%1
HF can also be classified as chronic HF or acute HF. Patients with chronic HF are those that have had HF for some time. If signs and symptoms of HF remain unchanged for at least 1 month, the condition is described at stable HF. However, chronic stable HF can deteriorate gradually. In contrast, acute HF is defined as the rapid onset of or change in signs and symptoms of HF. Immediate therapy is required for acute HF, which is a life-threatening condition. Urgent hospitalisation is a usual outcome with acute HF.1
Epidemiology
There are around 90,000 people in Ireland living with heart failure. Both the incidence and prevalence of heart failure increase with age. The majority of heart failure cases are in the over 65s2
AF, atrial fibrillation; HFrEF, heart failure with reduced ejection fraction; JVP; jugular venous pressure.
References
- European Heart Journal (2021) 42, 35993726 ESC GUIDELINES doi:10.1093/1. eurheartj/ehab368
- Global Heart Hub. Irish Heart Failure Barometer 2019. https://globalhearthub.org/wp-content/uploads/2019/05/HF_Barometer-Irish...
