For a trusted response in immune thrombocytopenia (ITP) patients
ITP patients can feel frustrated with their symptoms, often experiencing long periods of uncertainty and anxiety with delays to diagnosis and several, often-unsuccessful rounds of treatments.1–3
However, REVOLADE can offer your ITP patients rapid and sustained improvements that could help to alleviate disease burden4–6
The International Consensus Report (ICR) recommends a patient-centric approach through the following treatment goals:7
Prevent severe bleeding episodes
Optimise health-related quality of life (HRQoL)
Provide treatment with minimal toxicity
Maintain a platelet count of >20,000–30,000/μL for symptomatic patients
REVOLADE can help your ITP patients achieve these goals through responses that are:4,5
Platelet counts doubled as early as Week 15‡
- REVOLADE patients see an increase in platelet counts after as little as 1 week of treatment, with median platelet counts doubling from 16,000/μL to 36,000/μL
- From day 15 throughout treatment with REVOLADE, median platelet counts were between 53,000/μL (22,000-97,000) and 73,500/μL (32,000-131,000)5
- REVOLADE is also able to offer patients rapid relief from bleeding symptoms, with a ~50% reduction in the rate of clinically significant bleeding† as early as Day 15
What can a rapid response mean to your ITP patients?
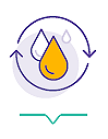
Improvements in platelet counts for ≥80% of patients4*
- Majority of REVOLADE patients (≥80%) are able to achieve a platelet count ≥50,000/μL, regardless of baseline platelet count [<30,000/μL, 81.5% (n=172/211); 30–50,000/μL, 98.1% (n=51/52); ≥50,000/μL, 92.3% (n=36/39)] or splenectomy status [splenectomised, 80% (n=92/115); non-splenectomised, 89.3% (n=167/187)]
- For ≥31 weeks, most (51%, n=126/248) REVOLADE patients are able to maintain platelet counts ≥50,000/μL; the majority of REVOLADE patients (55%, n=108/198) are also able to maintain platelet counts ≥30,000/μL for ≥91 consecutive weeks
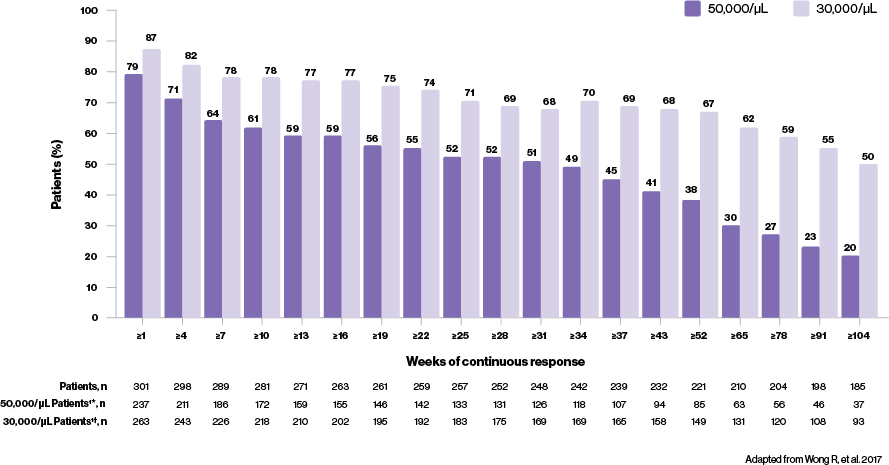
What can a consistent response mean to your ITP patients?
Thrombopoietin receptor agonists recommended for durable platelet responses8
- In the long-term RAISE study,‡ median platelet counts were maintained at ≥50,000/μL throughout 250 weeks of treatment4
- In addition to this, it has been shown that most patients can achieve a durable response with REVOLADE regardless of splenectomy status; 51% (n=19/37) of splenectomised and 66% (n=38/58) of non-splenectomised patients maintaining increased platelet counts5‡
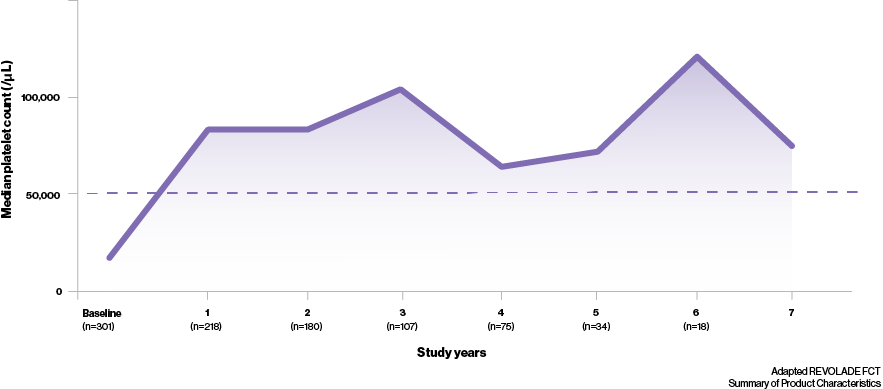
What can a durable response mean to your ITP patients?
TRUST REVOLADE to achieve the platelet response your patients can count on1–9
FCT, film-coated tablet; HRQoL, health-related quality of life; ICR, International Consensus Report; ITP, immune thrombocytopenia; WHO, World Health Organization.
*The phase III, open-label EXTEND study evaluated long-term safety and efficacy of REVOLADE in adults with ITP (defined as >6 months duration with baseline platelet counts <30,000/μL ) who have completed a previous REVOLADE study. The study reviewed more than 8 years of continuous treatment; 302 patient were enrolled and 135 (45%) completed the study, 60% were treated for at least 2 years and 35% at least 3 years.4
†Clinically significant bleeding defined as WHO grades 2–4 (grade 0, no bleeding; grade 1, petechiae; grade 2, mild blood loss; grade 3, gross blood loss; grade 4, debilitating blood loss).5
‡The phase III, double-blind, placebo-controlled RAISE study evaluated the response of ITP patients (defined as >6 months duration with baseline platelet counts <30,000/μL ) to REVOLADE over 6 months of treatment5
References:
-
Cooper N, et al. Burden of Disease in Immune Thrombocytopenia (ITP): The results for UK patients from the ITP World Impact Survey (I-WISh). Poster presented at the British Haematology Society Annual Meeting April 16–18, 2018. Liverpool. PO-022.
-
Cooper N, et al. Am J Hematol. 2021;96:188–198.
-
McCrae K. Cleve Clin J Med. 2011;78:358–373.
-
Wong R, et al. Blood. 2017;130:2527–2536.
-
Cheng G, et al. Lancet. 2011;377:393–402.
-
Mathias S, et al. Health Qual Life Outcomes. 2008;6:13.
-
Provan D, et al. Blood Adv. 2019;3:3780–3817.
-
Neunert C, et al. Blood Adv. 2019;3:3829–3866.
-
REVOLADE Summary of Product Characteristics.