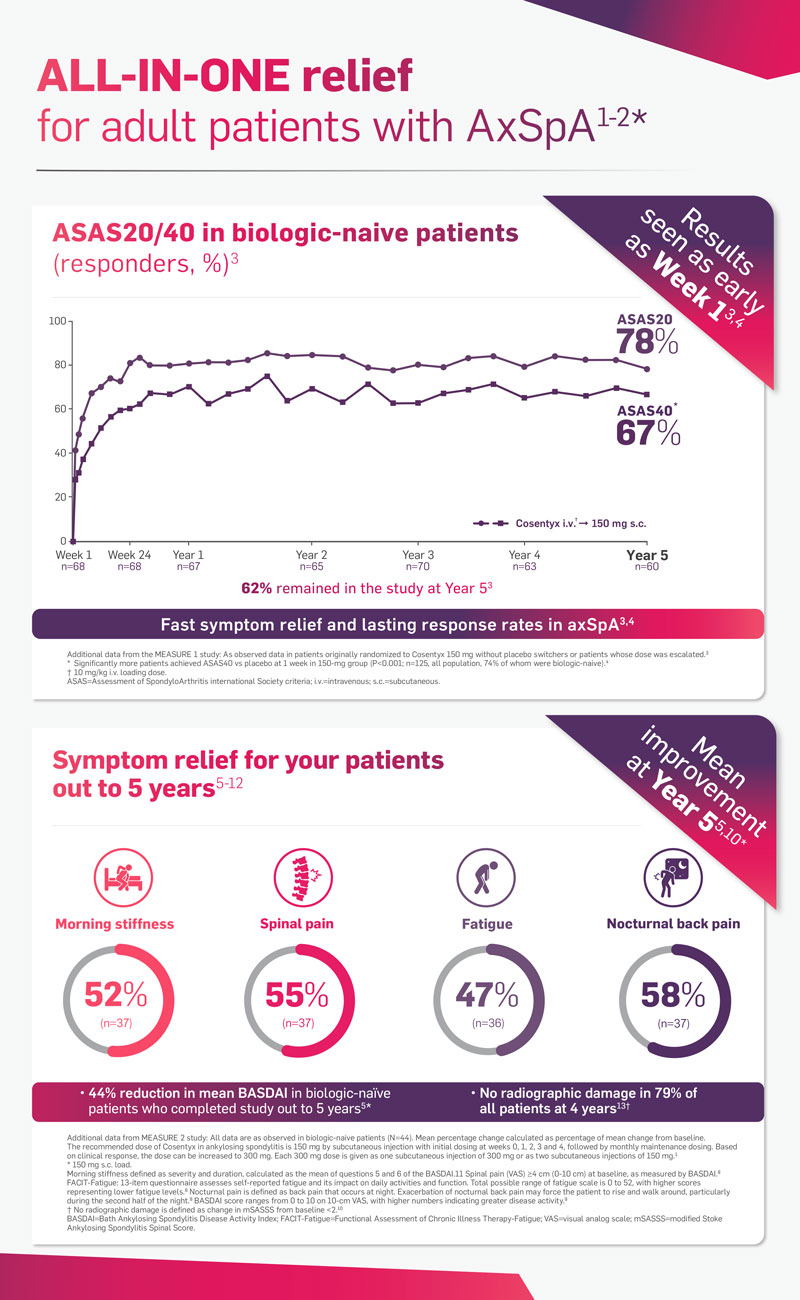
Why Cosentyx in axSpA?
Dive into the data below to see how Cosentyx could help your patients with axSpA
Take the next step
We’re here to support you with dedicated representatives up and down the country. Let us connect you with your local representative to discuss how you can get the most out of Cosentyx.
Or find out more using the links below...
- Cosentyx (secukinumab) Summary of Product Characteristics. Available at www.medicines.ie. Accessed November 2024
- Marzo-Ortega H et al. Arthritis Rheumatol. 2019;71(suppl 10). Abstract 1504.
- Baraliakos X et al. RMD Open. 2019;5(2):e001005.
- Baeten D et al. N Engl J Med. 2015;373(26):2534-2548.
- Novartis data on file. CAIN457F2310 Data Analysis Report. June 2019.
- Novartis data on file. CAIN457F2310 (MEASURE 2): Nocturnal Back Pain. January 2020.
- Marzo-Ortega H et al. Arthritis Care Res. 2017;69(7):1020-1029 and Supplementary Tables.
- Deodhar A et al. Clin Exp Rheumatol. 2019;37(2):260-269.
- Taurog JD et al. N Engl J Med. 2016; 375(13):1303.
- Zochling J. Arthritis Care Res (Hoboken). 2011;63(suppl 11):S47-S58.
- Novartis data on file. CAIN457F2310 (MEASURE 2): Nocturnal Back Pain. February 2021.
- Zochling J. Arthritis Care Res (Hoboken). 2011;63(suppl 11):S47-S58.
- Braun J et al. Rheumatology (Oxford). 2019;58(5):859-868.



