ENTRESTO Irish real-world evidence
ENTRESTO is indicated in adult patients for the treatment of symptomatic chronic heart failure with reduced ejection fraction.1
For full safety information, please refer to the Summary of Product Characteristics.1
Please see the below example of ENTRESTO use in the real-world setting.
Click on the links below to access individual cases.
Before looking through, please ensure you fully understand all prescribing considerations as per the Safety profile page.
Click on the links below to explore evidence for Entresto efficacy and its safety profile in the real-world setting:
1South of England – Retrospective comparison of 552 patients over 2 years3
2Northern Ireland – NYHA class improvement post treatment with ENTRESTO4
3West Midlands – Majority of patients prescribed adhere to NICE criteria5
4University Hospitals Coventry & Warwickshire - Retrospective evaluation of the clinical use of ENTRESTO in a large cardiovascular centre6
5West Suffolk Hospital – Rapid optimisation of ENTRESTO first line in newly diagnosed CHF7
6Cardiff – Improved cardiac function, symptoms and QoL post treatment with ENTRESTO8
1. South of England – Retrospective comparison of 552 patients over 2 years3
This study was published in Heart, an international peer reviewed journal from BMJ and BCS publishing. No conflicts of interest were reported.
Objective of the study
Investigate the extent to which the real-world use of ENTRESTO mirrors that of the PARADIGM-HF population and complies with guideline recommendations.
| Study | Background | Objective | Findings |
|---|---|---|---|
|
Real-world use of ENTRESTO in the South of England
|
Design: Retrospective comparison of n=552 patients started on ENTRESTO between 2017 and 2019 at 4 centres in the South of England with those from the treatment arm of the PARADIGM-HF study. In the final analysis, n=438 patients were included. |
Investigate the extent to which the real-world use of ENTRESTO mirrors that of the PARADIGM-HF population and complies with guideline recommendations. |
|
Cardiologists from the 4 South of England centres studied have prescribed ENTRESTO in a wide range of heart failure patients
NYHA class by cohort
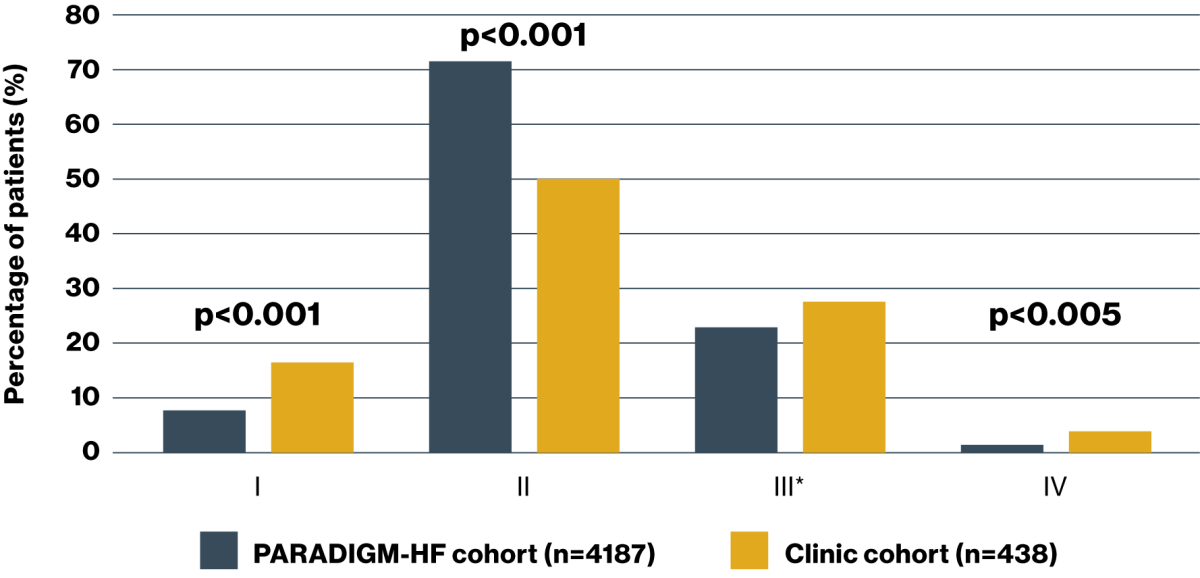
Adapted from Di Marco A, et al. 2020.9
*The proportion of patients who were NYHA class III within the PARADIGM-HF and clinic cohorts were described as ‘similar’, however the p value for this comparison was not clear in the source publication (p0.05).
Baseline characteristics of the South of England population
| Baseline characteristics | |||
|---|---|---|---|
| PARADIGM-HF cohort N=4187 | Clinic patients N=438 | p value | |
| Age (years) | 63.8 ± 11.5 | 68.7 ± 12 | <0.001 |
| Female sex | 879 (21.0) | 71 (20.3) | 0.01 |
| SBP mmHg | 122 ± 15 | 123 ± 20 | 0.18 |
| Heart rate – bpm | 72 ± 12 | 69 ± 12 | <0.001 |
| Creatinine – mg/dl | 1.13 ± 0.3 | 1.13 ± 0.3 | 1.0 |
| Clinical features | |||
| Ischaemic cardiomyopathy | 2506 (59.9) | 219 (50.0) | <0.001 |
| LVEF – % | 29.6 ± 6.1 | 29.9 ± 4.8 | 0.69 |
| Medical history | |||
| Hypertension | 2969 (70.9) | 156 (35.6) | <0.001 |
| Diabetes | 1451 (34.7) | 112 (25.6) | <0.001 |
| Numbers are total (%) or mean ± standard deviation | |||
92.5% of clinic patients had no exclusion criteria
- 4 had an eGFR <30 mL/min/1.73 m2 at baseline*
- 30 had an SBP <100 mmHg†
74% of clinic patients fulfilled inclusion criteria
- 68 were NYHA class I; 47 had an LVEF ≥35%
*ENTRESTO was not recommended in patients with end-stage renal disease, and concomitant use with aliskiren‐containing medicinal products in patients with diabetes mellitus or in patients with renal impairment (eGFR <60 ml/min/1.73 m2) was contraindicated. Use with caution in patients with severe renal impairment (eGFR <30 mL/min/1.73 m2). (See section 5.1 of the Summary of Product Characteristics). Starting dose of 24 mg/26 mg twice daily and slow dose titration (doubling every 3–4 weeks) are recommended in these patients.1,2
†Treatment should not be initiated unless SBP is ≥100 mmHg. Patients with SBP <100 mmHg were not studied.1,2
| Outcomes | |||
| PARADIGM-HF cohort N=4187 | Clinic patients N=438 | p value | |
| ENTRESTO discontinued | 746 (17.8) | 61 (13.9) | 0.04 |
| Dose of ENTRESTO at last assessment | 375 ± 71 mg | 297 ± 120* | <0.001 |
| Decline in renal function (50% or more in eGFR)‡ | 94 (2.2) | 18 (6.5)† | <0.001 |
| New offset AF | 84 (2.0) | 7 (1.6) | 0.55 |
| Elevated serum creatinine ≥2.5 mg/dl | 139 (3.3) | 12 (2.7) | 0.51 |
| Elevated serum creatinine ≥3.0 mg/dl | 63 (1.5) | 12 (2.7) | 0.05 |
| New onset cough | 474 (11.3) | 10 (2.3) | <0.001 |
| Angiodema | 19 (0.4) | 2 (0.5) | 0.99 |
| Symptomatic hypotension with SBP <90§ | 112 (2.7) | 7 (1.6) | 0.13 |
| Numbers are total (%) or mean ± standard deviation | |||
*Excludes patients where ENTRESTO was stopped.
†275 results only.
‡Use of sacubitril/valsartan may be associated with decreased renal function. The risk may be further increased by dehydration or concomitant use of NSAIDs. Down-titration should be considered in patients who develop a clinically significant decrease in renal function.1,2
§If hypotension occurs during treatment, temporary down-titration or discontinuation of sacubitril/valsartan is recommended.1,2
Download the ENTRESTO clinical trial summaries for more information

2. Northern Ireland – NYHA class improvement post treatment with ENTRESTO4
Novartis sponsored Br J Cardiol 2019;26(suppl 1): peer-reviewed journal. The sponsored supplement was initiated and funded by Novartis Pharmaceuticals UK Ltd. Editorial control, was retained by the authors and editors but Novartis reviewed the supplement for technical accuracy and compliance with relevant regulatory requirements before publication.
Objective of the study
Review of how ENTRESTO has been initiated by HF nurse specialists in Northern Ireland, the management of its side effects and its effect on patient outcomes.
| Study | Background | Objective | Findings |
|---|---|---|---|
|
Real-world experience and clinical data with ENTRESTO: a Northern Ireland perspective:4
|
The Southern Health and Social Care Trust in Northern Ireland has a nurse-led HF service, with 7 HF nurse specialists serving approximately 1500 patients. HF patients can be reviewed in either a domiciliary, clinic or acute setting, with nurses supported by consultant cardiologists, renal consultants, GPs and a cardiology pharmacist. The nurses also have access to cardiac investigations. HF nurse specialists in the Trust began to initiate patients on ENTRESTO in September 2016 following publication of the NICE guidance. |
To review how ENTRESTO has been initiated by HF nurse specialists in Northern Ireland, the management of its side effects and its effect on patient outcomes. |
|
Prescribing ENTRESTO
- Patients were prescribed ENTRESTO if they met the following criteria from the NICE guidance:
- NYHA class II or above
- LVEF ≤35%
- Already established on an ACEi or ARB
- No extra clinics were run, and no additional resources were allocated
- The majority of patients were initiated on ENTRESTO at the nurse-led HF clinics
- Prior to initiation, patients underwent a full clinical assessment by the HF nurse specialists, including medication history and review of renal and hepatic function
Experience with ENTRESTO
- 463 patients have been initiated on ENTRESTO in the Southern Health and Social Care Trust
- 31 patients (7%) discontinued ENTRESTO, mostly due to significant decline in renal function, symptomatic hypotension, or diarrhoea
- Careful assessment of volume status and diuretic dose pre-initiation is essential
- Regular contact with the HF nurse specialists, supported by other HCPs, often helped to avoid the need for withdrawal of therapy
- NYHA class improved in patients treated with ENTRESTO, most obviously in patients who have moved from NYHA class III to class II
- For some patients, this has meant that they no longer require referral for cardiac resynchronisation therapy
- One patient was removed from the transplant list
“We hope we have demonstrated that [ENTRESTO] can be appropriately initiated, titrated and managed in this [HF nurse specialist] setting.” – Donnelly E and Patton C, 20194
3. West Midlands – Retrospective multicentre study of 118 symptomatic chronic HFrEF patients in three UK hospital Trusts5
This study was published in the Journal of Prescribing Practice, after peer review and technical editing by the publisher. No conflicts of interest were reported.
Objective of the study
A retrospective multicentre study to explore real-life patient data regarding prescribing of ENTRESTO for symptomatic chronic HF patients in three hospitals, in accordance with national guidelines.
| Study | Background | Objective | Findings |
| To explore prescribing practices of ENTRESTO at three UK hospitals over a 6-month period in accordance with national NICE guidelines. |
Design: A multicentre retrospective study was conducted over a 6-month period of the use on ENTRESTO. The study proforma was adapted from NICE (2016a) guidelines. Patient information collected: NYHA functional class, LVEF, and prior HF medication. Data on ENTRESTO treatment was gathered: this included side effects, re-admission, discontinuation, and patient demographic data. In addition, blood pressure data, heart rate, eGFR, potassium and sodium levels were collected, as well as patients’ baseline medication (beta-blockers, mineralocorticoid antagonists, diuretics) and devices (implantable cardioverter defibrillator (ICD), cardiac resynchronisation therapy (CRT) and pacemakers (PPM)). |
|
|
Adherence to NICE guidance* was high across the 3 hospital cohorts studied
Patients prescribed ENTRESTO according to NICE guidance* across the total cohort studied (N=118)
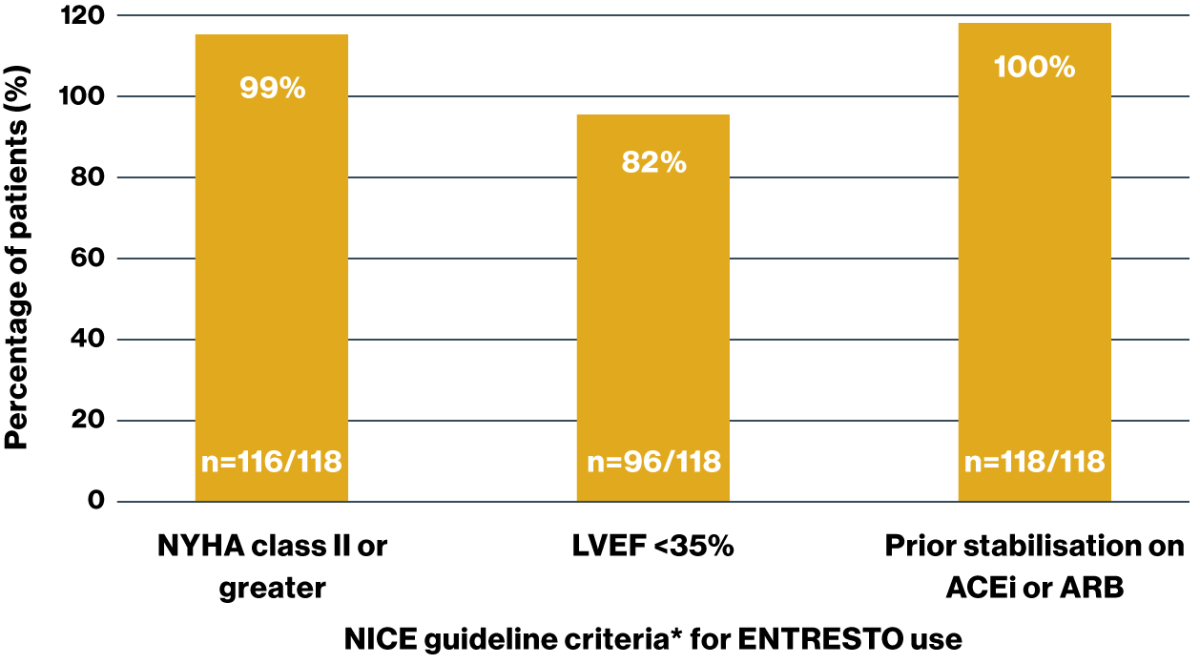
The majority (93%) of prescribers adhered to the 3 main NICE criteria for ENTRESTO prescribing
Substantially fewer patients were prescribed ENTRESTO than predicted by the NICE resource tool
Time to initiation and number of patients prescribed ENTRESTO varied across Trusts
![]()
HOSPITAL 1 (predicted uptake:† n=404 patients)
Prescribing uptake onto ENTRESTO
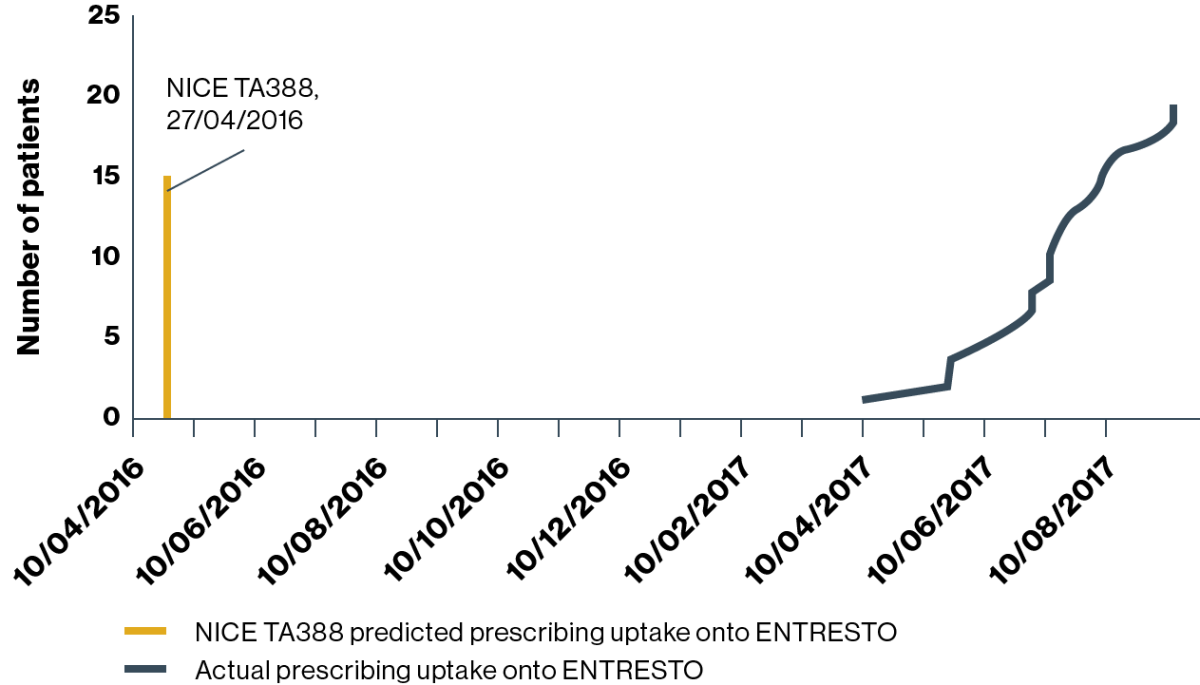
Adapted from Jalal Z, et al. 2019.4
Prescribing began at hospital 1 approximately 12 months after the NICE TA388 guideline for the use of ENTRESTO was published
![]()
HOSPITAL 2 (predicted uptake:† n=253 patients)
Prescribing uptake onto ENTRESTO
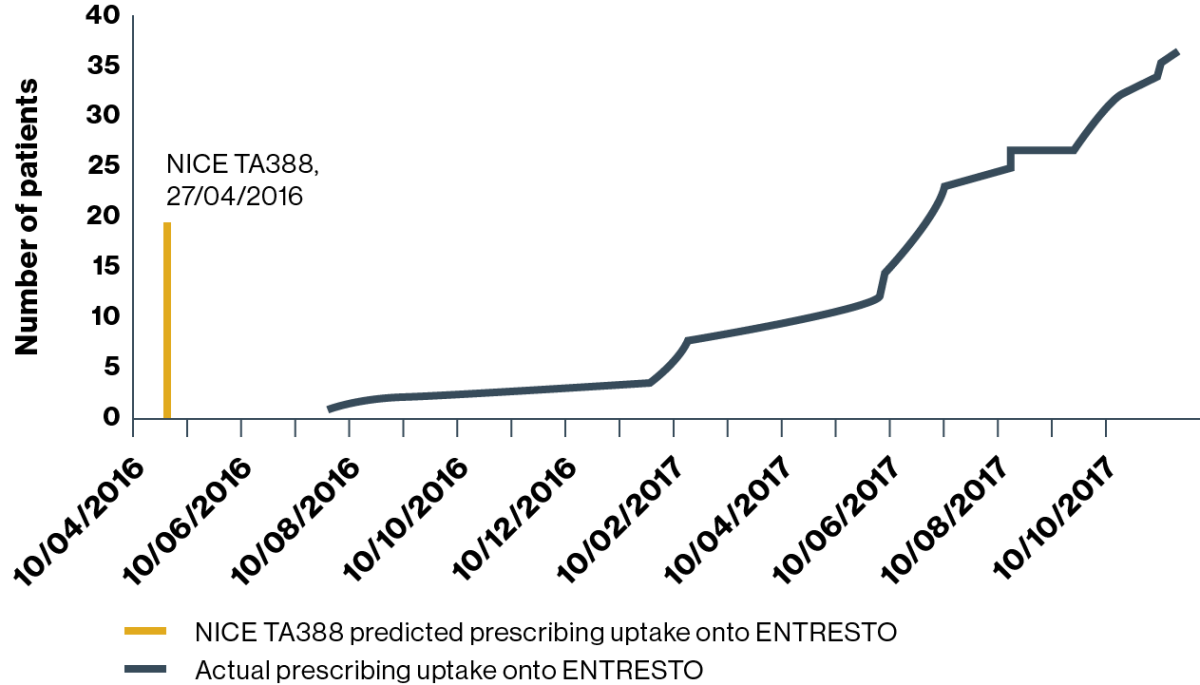
Adapted from Jalal Z, et al. 2019.4
Prescribers in hospital 2 started prescribing ENTRESTO in July 2016, three months after publication of NICE TA388
![]()
HOSPITAL 3 (predicted uptake:† n=494 patients)
Prescribing uptake onto ENTRESTO
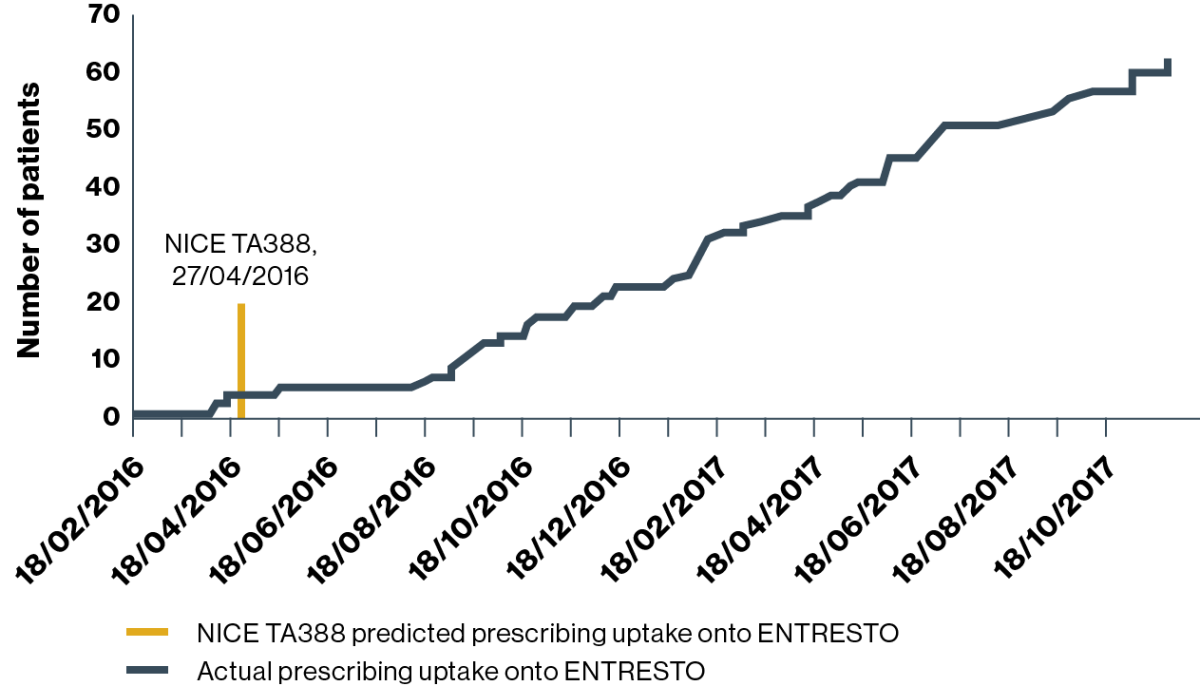
Adapted from Jalal Z, et al. 2019.4
There is potential to improve prescribing practices and recruiting of HF patients who meet the criteria for ENTRESTO
At hospital 3 the prescribing of ENTRESTO started in February 2016, two months before the NICE TA388 guideline was published
ENTRESTO real-world safety profile‡
- The main side effects of ENTRESTO mentioned in the NICE guideline include hypotension, hyperkalaemia and renal impairment.4,10 Across all Trusts studied, on average 65% (n=76/118) of patients within this study experienced one of the following side effects: dizziness, hypotension, renal impairment and fatigue.4
Rehospitalisation
The rate of rehospitalisation in hospital 1 was 5% (n1=20) compared with 3% in hospital 2 (n2=37) and 24% in hospital 3 (n3=61). Difficulties in breathing, shortness of breath, orthopnoea and paroxysmal nocturnal dyspnoea where reported reasons responsible for rehospitalisation in hospital 1. One patient in hospital 2 was discontined on ENTRESTO. The patient was hospitalised due to shortness of breath, chest pain and development of acute kidney injury (AKI). At hospital 3, 28 patients were seen in A&E during ENTRESTO treatment. However, only 15 cases could be identified to be categorically caused by HF-presenting complaints such as chest pain, shortness of breath and decompensated HF. Hospitals 1 and 2 had lower rehospitalisation rates associated with HF than the PARADIGM-HF study, where 12.8% of patients were rehospitalised. Hospital 3 had higher rates of rehospitalisation. These results cannot be comparable due to the large disparity in sample size; the PARADIGM-HF study recruited 8442 patients, with 4187 on ENTRESTO, compared to the 118 patients included in this study.4 Discontinuation None of the patients studied at hospital 1 discontinued their ENTRESTO medication. However, at hospital 2, there were three cases 8% (n2=37) where patients were discontinued on ENTRESTO. Of these, there were two cases of hypotension and one of AKI. At hospital 3, there were nine cases 15% of discontinuation (n3=61); three were due to death (one death was due to AKI), five from adverse events and one due to a prescriber error. Of those where discontinuation was due to adverse events, one was due to an AKI, one had kidney impairment, two patients had a potential allergic reaction and one patient had recurrent hypotension that led to discontinuation of ENTRESTO. In the PARADIGM-HF trial, there were few reports of adverse events that lead to discontinuation 10.7% (P=0.03). These results cannot be comparable due to the large disparity in sample size; the PARADIGM-HF study recruited 8442 patients, with 4187 on ENTRESTO, compared to the 118 patients included in this study.4
The most commonly reported AEs with ENTRESTO in the PARADIGM-HF trial were hypotension (17.6%), hyperkalaemia (11.6%) and renal impairment (10.1%).1,2 To review the side effect profile of ENTRESTO click here.
Discontinuation
None of the patients studied at hospital 1 discontinued their ENTRESTO medication. However, at hospital 2, there were three cases 8% (n2=37) where patients were discontinued on ENTRESTO. Of these, there were two cases of hypotension and one of AKI. At hospital 3, there were nine cases 15% of discontinuation (n3=61); three were due to death (one death was due to AKI), five from adverse events and one due to a prescriber error. Of those where discontinuation was due to adverse events, one was due to an AKI, one had kidney impairment, two patients had a potential allergic reaction and one patient had recurrent hypotension that led to discontinuation of ENTRESTO. In the PARADIGM-HF trial, there were few reports of adverse events that lead to discontinuation 10.7% (P=0.03). These results cannot be comparable due to the large disparity in sample size; the PARADIGM-HF study recruited 8442 patients, with 4187 on ENTRESTO, compared to the 118 patients included in this study.4
*NICE guidelines recommend ENTRESTO as an option for treating symptomatic chronic HFrEF, only in people: with NYHA class II to IV symptoms and; with a LVEF ≤35%; who are already taking a stable dose of ACEi or ARBs. Treatment with sacubitril/valsartan should be started by a heart failure specialist with access to a multidisciplinary heart failure team. Dose titration and monitoring should be performed by the most appropriate team member as defined in the NICE guideline on chronic heart failure in adults: diagnosis and management.10
†Based on number of patients anticipated to have met the treatment criteria detailed in NICE TA388 each year.
‡Sample size in this study was small and no clear conclusion could be made due to inconsistency in the documentation of side effects in the 3 hospital databases.
4. University Hospitals Coventry & Warwickshire – Retrospective evaluation of the clinical use of ENTRESTO in a large cardiovascular centre6
Br J Cardiol 2019;26(suppl 1):
Peer-reviewed journal. The sponsored supplement was initiated and funded by Novartis Pharmaceuticals UK Ltd. Editorial control, was retained by the authors and editors but Novartis reviewed the supplement for technical accuracy and compliance with relevant regulatory requirements before publication.
Objective of the study
Evaluation of the clinical use of ENTRESTO in a large cardiovascular care centre, in terms of the morbidity and mortality, tolerability and up-titration to its guideline-mandated target dose (97/103 mg BD) in patients switched from ≥4 weeks ACEi/ARB therapy.
| Study | Background | Objective | Findings |
|
Early clinical experience with ENTRESTO from a large UK tertiary centre:
|
Design: A real-world, retrospective evaluation of the effects of ENTRESTO in 140 patients aged ≥18 years with HFrEF (≤35%), NYHA class II–IV, eGFR >30 mL/min/1.73 m2 and ≥4 weeks’ previous treatment with ACEi/ARB. Patients were studied from April 2016 to July 2017 in a nurse-led, MDT-backed HF clinic. |
To evaluate the clinical use of ENTRESTO in a large cardiovascular care centre, in terms of morbidity and mortality, tolerability and up-titration to its guideline-mandated target dose (97/103 mg BD) in patients switched from ≥4 weeks ACEi/ARB therapy. |
|
Baseline characteristics
| Baseline characteristics | |
|---|---|
|
Male |
108 (77%) |
|
Mean age (range), years |
67 (29–89) |
|
Mean LVEF, % (range) |
23 (8–35) |
| NYHA class | |
|
II |
74 (53%) |
|
III |
65 (46%) |
|
IV |
1 (1%) |
| Comorbidities | |
|
Ischaemic heart disease |
46 (33%) |
|
Atrial fibrillation |
53 (38%) |
|
Diabetes mellitus |
36 (26%) |
|
Hypertension |
49 (35%) |
|
Chronic kidney disease |
39 (28%) |
| Medications | |
|
ACEi |
103 (73%) |
|
ARB |
38 (27%) |
|
Beta blocker |
135 (96%) |
|
MRA |
96 (69%) |
|
Loop diuretics |
104 (74%) |
| Devices | |
|
Cardiac resynchronisation therapy* |
22 (16%) |
|
Implantable cardioverter/defibrillator |
8 (6%) |
*Defibrillator/pacemaker.
Data shown are number (%), except where indicated.
| Up-titration of ENTRESTO in 140 patients initiated on this treatment | |
| Event* | N(%) |
|---|---|
|
Up-titration achieved to 97/103 mg BD |
77 (55%) |
|
Reduction in loop diuretic dosage |
30 (27%) |
|
ENTRESTO withdrawn for adverse events |
11 (8%) |
|
MRA dose reduced due to hyperkalaemia |
2 (2%) |
|
MRA stopped due to hyperkalaemia |
1 (1%) |
|
Postural hypotension with drop of SBP >10 mmHg |
44 (31%) |
*Treatment should not be initiated if the serum potassium level is >5.4 mmol/l or SBP is ≥100 mmHg. If either hypotension or hyperkalaemia occurs during treatment discontinuation should be considered. Please refer to the ENTRESTO GB and NI SmPC for full guidance.1,2
Up-titration to the target dose was achieved by 55% (n=77/140) patients
- ENTRESTO was not tolerated by 11 (8%) patients, mainly due to symptoms of postural hypotension
- Mean SBP fell noticeably between the second and third study visits and remained stable thereafter
- Postural hypotension (SBP reduction >10 mmHg) preventing up-titration to the target dose was observed in 31% (n=44/140) patients at clinic follow-up
Deterioration of renal function in a small proportion of patients did not prevent up-titration to target dose*
- eGFR deteriorated by >10 mL/min/1.73 m2 in n=15 (10.7%) patients. No progressive impairment of renal function was observed
- Five of the fifteen patients achieved up-titration to the target dose
Entresto resulted in low real-world HF mortality compared to PARADIGM-HF
| Clinical outcome | N(%) |
|
All-cause mortality at 6 months (n=140) |
5 (4%)† |
| All-cause mortality at 1 year (n=68) | 5 (7%) |
| HF admissions at 6 months (n=140) | 8 (6%) |
| HF admissions at 1 year (n=68) | 4 (6%) |
*Use of ENTRESTO may be associated with decreased renal function. The risk may be further increased by dehydration or concomitant use of NSAIDs. Down-titration should be considered in patients who develop a clinically significant decrease in renal function. ENTRESTO was not recommended in patients with end-stage renal disease, and concomitant use with aliskiren‐containing medicinal products in patients with diabetes mellitus or in patients with renal impairment (eGFR <60 ml/min/1.73 m2) was contraindicated. Use with caution in patients with severe renal impairment (eGFR <30 mL/min/1.73 m2). Please refer to the ENTRESTO GB and NI SmPC for full guidance.1,2
†Two related to HF, three not related to HF.
Patients demonstrated improved LVEF and/or symptomatic benefit following ENTRESTO treatment
- ENTRESTO treatment resulted in an improvement of at least one NYHA class for 31% (n=44/140) patients
- 66% of patients (n=23/34 patients who underwent repeat LVEF evaluation post-ENTRESTO treatment) demonstrated improved LVEF with a mean increase from 19% to 27% following ENTRESTO
Of 140 patients treated with ENTRESTO, 7 achieved a normal LVEF of ≥55% from a baseline of severe LV systolic impairment (LVEF ≤35%)5
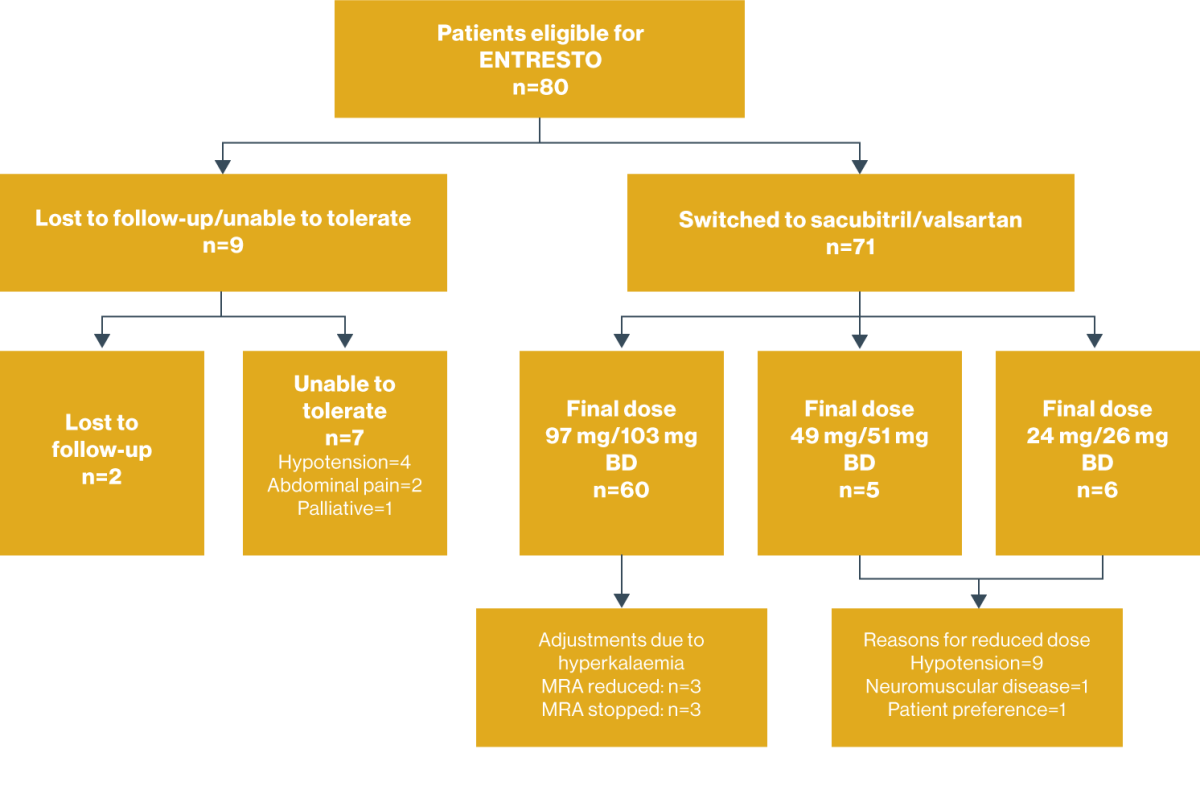
5. West Suffolk Hospital – Optimisation of ENTRESTO first line in newly diagnosed CHF7
This is unpublished data taken from a Master’s thesis. It has not been peer reviewed and should be treated as such. No conflicts of interest were reported.
Objective of the audit
Identification of new HF patients who have been or are being initiated on ENTRESTO without prior ACEi/ARB treatment and to assess the feasibility and safety of ENTRESTO dose optimisation.
| Study | Background | Objective | Findings |
|---|---|---|---|
|
Audit of newly diagnosed patients initiated with ENTRESTO:
|
At West Suffolk Hospital, all patients identified as eligible for ENTRESTO were initiated on treatment. Design: Combined retrospective and prospective audit of n=21 patients treated at a DGH HF service. |
To identify new HF patients who have been or are being initiated on ENTRESTO without prior ACEi/ARB treatment and to assess the feasibility and safety of ENTRESTO dose optimisation. |
|
| Criteria for commencing de novo ENTRESTO |
|
Echocardiogram confirming EF <35% |
|
Symptomatic heart failure NYHA class II–IV |
|
No previous exposure to ACEi or ARB |
|
BP systolic ≥100 mmHg |
|
At least moderate kidney function (eGFR >30 mL/min/1.73m2) |
|
No contraindication to ENTRESTO |
|
Clinically stable – no IV vasodilators or increase in IV diuretics in prior 6 hours; no IV inotrope in prior 24 hours |
Optimisation of ENTRESTO dose was achieved in 43% (n=9/21) patients initiated on ENTRESTO without prior ACEi/ARB treatment
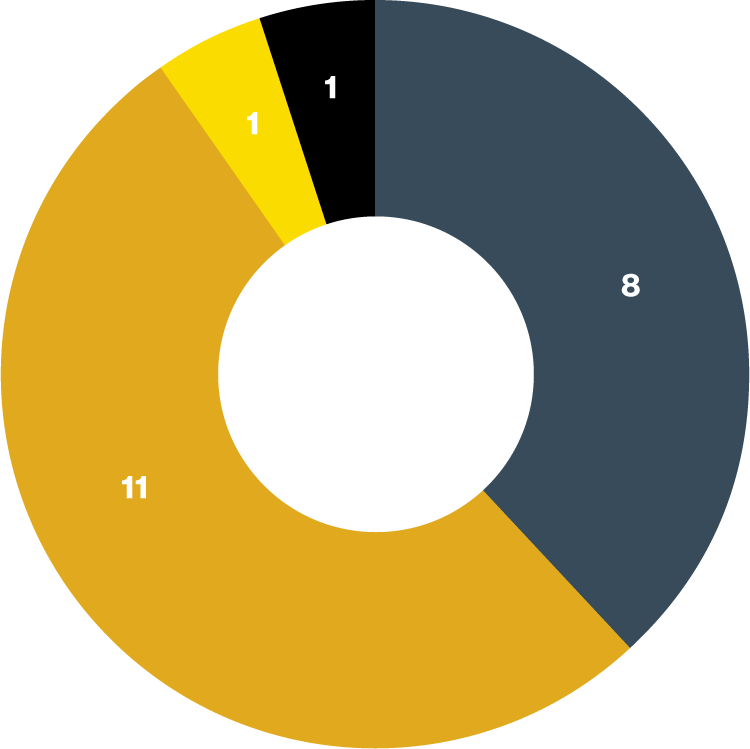
Adapted from Pugh J, et al. 2020.6
ENTRESTO dose optimised at initiation dose of 24 mg/26 mg, BD
ENTRESTO dose optimised at maximum dose of 97 mg/103 mg, BD
ENTRESTO dose optimisation not achieved, receiving 49 mg/51 mg BD
ENTRESTO stopped due to marked hypotension
Significant alteration of either MRA or beta-blocker medication delayed ENTRESTO titration for 11/21 patients. All patients achieved ENTRESTO dose optimisation within 6 months.
All patients initiated on ENTRESTO had blood pressure and renal function checked at Week 2, and, if receiving up-titrated ENTRESTO, Week 4
The following safety issues were encountered in this study:
- One patient stopped ENTRESTO at Week 4 due to symptomatic hypotension
- Of the 21 patients studied, 5 experienced >20% reduction in eGFR at Week 4:*
- 2/5 cases were sustained for the duration of the 6-month data collection period
- 2/5 cases improved to only a 10–15% decline in eGFR by the 6-month follow-up
- 1/5 cases completely resolved by the 6-month follow-up
Design: Combined retrospective and prospective audit of n=21 patients treated at a DGH HF service.
*Use of ENTRESTO may be associated with decreased renal function. The risk may be further increased by dehydration or concomitant use of NSAIDs. Down-titration should be considered in patients who develop a clinically significant decrease in renal function. ENTRESTO was not recommended in patients with end-stage renal disease, and concomitant use with aliskiren‐containing medicinal products in patients with diabetes mellitus or in patients with renal impairment (eGFR <60 ml/min/1.73 m2) was contraindicated. Use with caution in patients with severe renal impairment (eGFR <30 mL/min/1.73 m2). For full safety information see the GB and NI SmPC.1,2
6. Cardiff – Improved cardiac function, symptoms and QoL post treatment with ENTRESTO8
This study was published in the BMJ Open Heart Journal, and was externally peer reviewed. The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors. One of the authors, ZY, has received lecture fees and research funding from Novartis.
Objective of the study
Descriptions of the real-world experience of switching stable and optimally medicated patients with HFrEF to ENTRESTO with respect to quality of life and echocardiographic outcomes, and safety.
| Study | Background | Objective | Findings |
|---|---|---|---|
|
Real-world treatment switching to sacubitrll/valsartan in patients with heart failure with reduced ejection fraction:
|
Design: Retrospective cohort study of 80 consecutive patients with a diagnosis of stable HFrEF (defined as symptoms and/or signs of HF, NYHA functional classification of ≥II and a LVEF of ≤35% measured by echocardiography) from June 2017 to May 2019. Patients' clinical assessment, biochemistry, echocardiography and QoL were compared pre-treatment switching and post-treatment switching. |
Describe the real-world experience of switching stable and optimally medicated patients with HFrEF to ENTRESTO with respect to quality of life and echocardiographic outcomes, and safety. |
|
*ENTRESTO was not recommended in patients with end-stage renal disease and concomitant use with aliskiren‐containing medicinal products in patients with diabetes mellitus or in patients with renal impairment (eGFR <60 ml/min/1.73 m2) was contraindicated. Use with caution in patients with severe renal impairment (eGFR <30 mL/min/1.73 m2).1,2
Limitations of the study:
Small sample of sequential patients with short follow-up.
- Young age group (mean age of 64) may preclude extrapolation to an older cohort, who are more likely to have more advanced disease with a range of comorbidities, such as decline in renal function or autonomic dysfunction.
- Lack of comparator group in the study. Patients were already treatment optimised prior to sacubitril/valsartan switch so effectively acted as his or her own control.
ENTRESTO resulted in significant improvements in blood pressure, NYHA score and QoL (MLHFQ) in patients with stable HFrEF following a switch from ACEi/ARB to ENTRESTO
| n | Pre-switching | Post-switching | Mean difference (SD) | 95% CI | p value | |
|---|---|---|---|---|---|---|
| NYHA score |
71 |
2.3 |
1.9 |
–0.4 (0.63) | –0.6 to –0.2 | <0.001 |
| MLHFQ score |
33 |
46 |
38 |
–9 (19) | –15 to –2 |
0.016 |
| SBP (mm Hg) |
68 |
123 |
112 |
–10 (14) | –14 to –7 |
<0.001 |
| DBP (mm Hg) |
68 |
72 |
68 |
–4 (10) | –6 to –1 |
0.004 |
Significant improvements in cardiac structure and function were observed with ENTRESTO in patients with stable HFrEF following a switch from ACEi/ARB to ENTRESTO
| n | Pre-switching | Post-switching | Mean difference (SD) | 95% CI | p value | |
|---|---|---|---|---|---|---|
| LVEF (%) |
49 |
26 |
33 |
7 (10) | 4 to 10 |
<0.001 |
| LVESD (cm) |
37 |
5.2 |
4.9 |
–0.3 (0.8) | –6 to –0.08 |
0.013 |
| LVEDD (cm) |
48 |
6.0 |
5.7 |
–0.3 (0.7) | –0.5 to –0.1 | 0.042 |
ENTRESTO treatment was not associated with a significant change in renal function in patients with stable HFrEF following a switch from ACEi/ARB to ENTRESTO
| n | Pre-switching | Post-switching | Mean difference (SD) | 95% CI | p value | |
|---|---|---|---|---|---|---|
| K+ (mmol/L) |
71 |
4.6 |
4.7 |
0.1 (0.40) | –0.01 to 0.20 |
0.054 |
| Creatinine (µmol/L) |
71 |
95 |
97 |
2 (14) | –1 to 6 |
0.17 |
| eGFR (mL/min/1.73 m2) |
71 |
69 |
67 |
–2 (13) | –5 to 1 | 0.23 |
Consistent with the PARADIGM-HF trial, the most common symptomatic adverse event was hypotension
-
In PARADIGM-HF hypotension occurred in 740 patients (17.6%) on ENTRESTO vs 506 patients (12.0%) on enalapril.
Adapted from Ganesananthan S, et al. 2020.7
ACEi, angiotensin converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BD, twice daily; BP, blood pressure; bpm, beats per minute; CI, confidence interval; CKD, chronic kidney disease; DBP, diastolic blood pressure; DGH, district general hospital; EF, ejection fraction; eGFR, estimated glomerular filtration rate; GP, general practitioner; HCP, healthcare professional; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HFrLVEF, heart failure with reduced left ventricular ejection fraction; IV, intravenous; K+, potassium; LV, left ventricular; LVEDD, left ventricular end diastolic diameter; LVEF, left-ventricular ejection fraction; LVESD, left ventricular end systolic diameter; MDT, multidisciplinary team; MLHFQ, Minnesota Living with Heart Failure Questionnaire; MRA, mineralocorticoid receptor antagonist; NICE, National Institute for Health and Care Excellence; NYHA, New York Heart Association; PARADIGM-HF, Prospective Comparison of ARNI with ACEi to Determine Impact on Global Mortality and Morbidity in Heart Failure; QoL, quality of life; SBP, systolic blood pressure; SD, standard deviation.
References:
- ENTRESTO® Summary of Product Characteristics. Accessed on May 2024 www.medicines.ie.
- Pharithi RB, McDonald K. Response rate to Sacubitril/Valsartan in the community. Is there a clear Phenotype? ESC Heart Failure Congress 2019. Poster Presenta (P996).
- Mannion T, O'Connor L, Kelly O Haire J, 0 Gara G, McAdam BF. Effect of therapy with ARNI on Cardiac remodelling, functional and biomarker responses in selected cohort of patients with HF and influence of aetiology, gender and medication dose, ESC Heart Failure 2019. Poster Presentation (P394).
- McGovern L, Caples N, Cronin E, Asgedom S, Owens P. A single centre experience sacubitril/valsartan - A novel treatment for heart failure. ESC Poster presentation.
