Even when patients do not exhibit apparent symptoms, cardiac damage is constantly occurring1
Modest changes in LVEF may be clinically important; the adjusted risk of CV-related mortality increases two-to eight-fold beyond a >10-unit decline in LVEF (P<0.001).2
![]()
deterioration for cardiac myocytes in failing human hearts compared with healthy hearts3
![]()
approximately 90,000 people in Ireland live with Heart Failure (HF) with 10,000 cases diagnosed each year4,10
![]()
greater risk of death in the first month after just one HF hospitalisation in comparison with non-hospitalised patients5,6
![]()
of HFrEF patients may experience sudden cardiac death, even when their symptoms have not worsened7

ENTRESTO is indicated in adult patients for the treatment of symptomatic chronic heart failure with reduced ejection fraction.9
CV, cardiovascular; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; MoA, mode of action; MRA, mineralocorticoid receptor antagonist; QoL, quality of life.
References:
- Mann DL, Bristow MR. Circulation 2005;111(21):2837–2849.
- Strange G, et al. Eur J Heart Fail 2021;23(4):555–563.
- Konstantinidis K, Whelan RS, Kitsis RN. Thromb Vasc Biol 2012;32(7):1552–1162.
- Cost of Heart Failure Report, December 2015, Data on file
- Solomon SD, et al. Circulation 2007;116(13):1482–1487.
- Okumura N, et al. Circulation 2016;133(23):2254–2262.
- Packer M. Eur Heart J 2020; (41)1757–1763
- Health Policy Partnership, Heart Failure Barometer Report, 2019, Data on File.
- ENTRESTO Summary of Product Characteristics. Accessed on May 2024 at www.medicines.ie
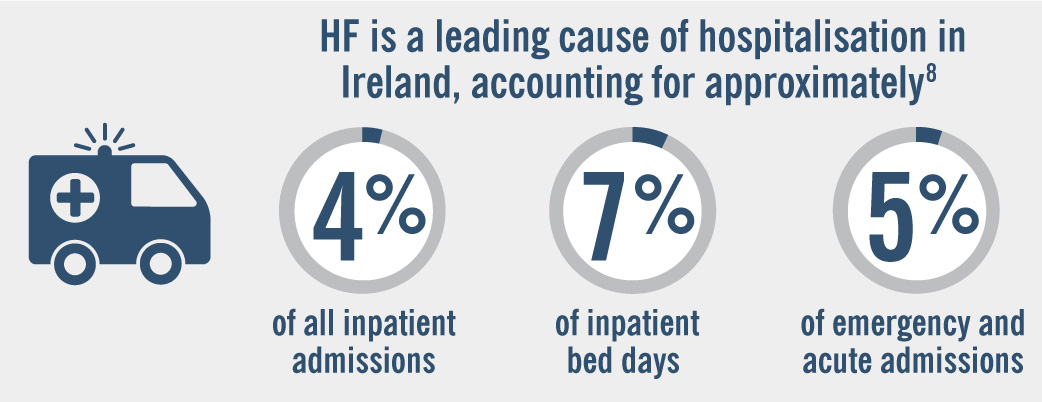
- Healthcare Pricing Office (2021) Activity in Acute Public Hospitals in Ireland Annual Report, 2020. Dublin. Health Service Executive. Accessed April 2021 at http://www.hpo.ie/latest_hipe_nprs_reports/HIPE_2020/HIPE_Report_2020.pdf

